

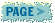
2.1 Post-harvest biodeterioration
2.8 Storage systems for cassava
2.1 Post-harvest biodeterioration
Cassava roots, when left attached to the main stem, can remain in the ground for several months without becoming inedible; farmers do often leave cassava plants in the field as a security against drought, famine or other unforeseen food shortage. It is from this property that cassava has earned its name as a 'famine reserve crop'. However, once the roots have been harvested, they start deteriorating within 2 to 3 days, and rapidly become of little value for consumption or industrial applications.
Two types of deterioration are known to occur. The first to appear -- therefrom named 'primary deterioration' -- consists of physiological changes characterised by an internal root discoloration called vascular streaking or vascular discoloration (Averre, 1967). It is displayed as blue-black or brownish occlusions and chemical deposits. The time to onset of primary deterioration and the rate at which it progresses, the intensity, pattern and distribution of the discoloration varies between cultivars and roots of the same plant. Some varieties deteriorate so fast they become inedible 24 hours after harvest (Booth, 1976) while others have been reported to stand for 7 to 11 days at room temperature without any sign of discoloration (Montaldo, 1973). From a biochemical point of view, primary deterioration of cassava roots is associated with a conversion of some of the starch to sugars (Booth et al 1976), an accumulation of cyanogenic glucosides, a decrease in linamarase activity (Kojima et al. 1983), and the onset of a number of enzymatic reactions leading to the accumulation of coloured compounds (Wheatley and Schwabe, 1985).
There is a strong association between the onset of primary deterioration and the occurrence of various forms of mechanical damage. Due to the nature of harvesting and handling operations, mechanical damage is unavoidable; cutting the root off the plant creates a wound; digging utensils may cut or scrap the roots. Breaking off of the root tips and bruising do occur during transportation and handling. Wounds and bruises are the triggers of primary deterioration. Booth (1976) found that primary deterioration was essentially a wound response being initiated near the region of mechanical damage; unlike in other storage organs (e.g. sweet potato), the response is not localised at the surface, but spreads down the root. Wounds and bruises also constitute points of entry for micro-organisms leading to the second stage of cassava root spoilage, known as "secondary deterioration".
Secondary deterioration is induced by micro-organisms that cause rotting. Two types of rot have been identified. Under aerobic conditions, fungi cause a dry rot which results in discoloration and a slight rise in acidity; under anaerobic conditions, bacterial activity (mainly due to Bacillus sp.) predominates, giving rise to rapid development of acidity (Ingram and Humphries, 1972). Most of these organisms behave as wound pathogens and infect roots through the sites of injury, and this usually occurs after primary deterioration has set in, and the roots have already lost their appeal to consumers.
Cassava roots can be harvested at any time of the year. Some farmers harvest as early as six months after planting while others may leave the crop for 18 to 24 months. The food quality of roots, particularly the starch content, increases with time up to an optimal period of 12 to 15 months after planting, after which there is a loss of quality, mainly due to increased lignification. During the dry season, cassava usually drops its leaves. At the onset of rains, a dramatic shift in root quality takes place, probably due to a remobilization of starch towards new leaf formation: the mealy texture of boiled cassava root is often lost, and roots can no longer be used for this purpose.
Harvesting cassava roots is usually done by hand; it is easy if the soil are sandy or during the rainy season. In heavier soils or during the dry season, harvesting usually requires digging around the roots to free them and lifting the plant. To facilitate lifting, the plant is usually cut down about 30 to 50 cm above ground. The protruding stem is used to lift the roots out of the ground. While lifting, care should be taken not to break the roots, as this will lead to losses if broken roots are not retrieved from the soil and to contamination that may evolve into spoilage.
After clearing the land, harvesting is the most labour-intensive operation, and agricultural engineers have sought to mechanise it. Mechanical harvesting of cassava is difficult because of the non-uniform geometry of the roots in the ground. Nevertheless a few cassava harvesters have been designed and some are in operation, mostly by large-scale farmers. The cost of mechanical harvesting is too high for resource-pour farmers.
Young leaves and shoots of cassava are also harvested to be consumed as vegetables and may be as important as roots for generating cash income. Excessive harvesting of the leaves can have a negative effect on the yield of roots. However, it has been shown in D.R. Congo where cassava leaves are extensively commercialised, an optimal leaf harvesting schedule of once every one or two months will result in higher overall returns for the farmer (Lutaladio and Ezumah, 1981).
2.2.1 Methods
The Amerindians, who first cultivated cassava, also devised numerous processing techniques not only to increase the palatability of cassava and to extend its shelf life, but also to decrease its cyanogenic potential. Today, a great diversity of processing methods are found in the various parts of the world where cassava is consumed (Lancaster et al.,1982). They consist of combinations of the primary processing steps described below.
Peeling
The first step in processing cassava roots is often to remove the peel; this result in a great reduction the cyanogenic potential of the raw material, because the peel represents about 15 percent of the weight of the root, and its cyanogen content is usually 5 to 10 times greater than that of the root parenchyma. However, the peel also contains large amounts of the enzyme linamarase which is important in the detoxification of cassava during processing. For instance, grinding cassava roots without removing the peel, as is done in the manufacture of the Brazilian farinha, ensures an almost total elimination of cyanogens from cassava.
Peeling is usually done by hand using a knife; the process is slow and labour-intensive, averaging 25 kg per man-hour, but it gives the best results. The Post-harvest Engineering Unit of IITA has developed a cassava peeling tool that is simple, can be fabricated locally and gives minimum peeling losses (See Figure 4a). Mechanical peelers are generally wasteful and with low efficiency. All solutions, including chemical ones that have been developed so far have proved rather impractical. For several years more, peeling cassava will remain as a source of employment and income for rural dwellers, particularly if cassava-based agro-industries develop around cassava farms.
Figure 4a: Cassava peeling tool
Boiling
Cassava is often consumed as a vegetable after boiling for 15 to 45 minutes. Some cassava varieties give a soft, mealy and easy to mash boiled roots. In some parts of Africa, after boiling, the roots from these types of varieties are pounded into a smooth paste called fufu. Other varieties give roots which when boiled remain hard and are waxy; these cannot be pounded into fufu. IITA and several African countries have breeding programs to develop the mealy-type cassava varieties. Wherever boiled cassava is consumed, mealiness is the main quality characteristic.
Size reduction: chipping and grating
The size of cassava roots is usually too large to process and is usually reduced prior to further processing. At the home level, cassava roots are chipped manually using a knife. This process is slow and produces large and irregular chips that take 3 to 7 days to dry and impart a sour and musty taste, actually preferred by some consumers, to the food made from the dry chips. Mechanical chippers have the advantage of producing smaller and uniform chips that dry rapidly. The drying rate depends on the geometry of the chips and the amount of chips per unit of drying surface. Flat chips tend to stick to each other and reduce the flow of removal of moisture between chips that are stuck together. IITA has designed a low-cost chipper that produces 'finger' chips of about 5mm thickness and a length depending on the size of the cassava roots (IITA, 1996). These chips can dry after 6 to 8 hours of exposure to the sun. When manually operated, the chipper has a capacity of 60-70 kg/hr; but an electric, gasoline or diesel engine can power it with a capacity of 1000kg/hr.
The initial step of several processes for the preparation of foods from cassava is pulping, either by grating or by crushing freshly harvested cassava roots. Examples of foods prepared in this fashion are gari in Nigeria, farinha de mandioca in Brazil, cassava bread in various countries of Latin America and the Caribbean islands (Lancaster et al., 1982). Cassava starch extraction is also carried on after pulping. The pulping process results in the disintegration of cassava tissues, which favours the contact between linamarase and the cyanogenic glucosides. Processes that begin with pulping usually result in the greatest detoxification of the final product. This is the case of the farinha and gari processes, and of the starch extraction process. Once the pulp is obtained, it is usually squeezed to remove the juice. The remaining cake is further processed into food. The juice is discarded, or in some instances, it is used to prepare sauces or beverages (Lancaster et al., 1982). To make starch, the pulp is extensively washed with water to separate the starch granules from the soluble component of the pulp.
Pulping is the first step in the preparation of gari and attiéké (West Africa), farinha (Brazil, Caribbean). In Nigeria, the process of grating cassava has been widely mechanised; there are many types of grating machines to choose from. IITA has also developed a grating machine which can be manually operated or equipped with an engine (See Figure 5). The manual grater has a capacity of about 30 kg/hr, while the motorised grater has a capacity of 800 kg/hr. Young roots are usually easier to process, while roots from plants that are more than 18 months old require a longer time to grate because they are generally more fibrous and oppose a greater resistance to the grating process (IITA, 1996).
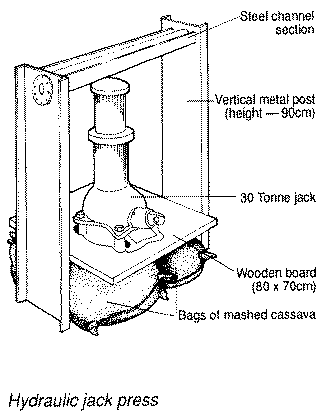
Figure 5. Equipment used in simple cassava processing
Pressing
After grating cassava, the next processing step is generally pressing the grated pulp to reduce its moisture content. The Amerindians who have been processing cassava for two millennia developed an ingenious press shaped like a long thin basket-weave tube called ´tipiti'. The tipiti would be filled with cassava mash, hung on a branch of a tree and stretched from the bottom; its volume would reduce and water would be squeezed out of the mash. In Africa, people used heavy stone placed on top of bags or baskets filled with cassava mash. More recently, screw presses and jack presses (See Figure 5) are used for greater efficiency and speed. In Brazil where grating and pressing cassava have been industrialised, hydraulic presses providing pressures of up to 25 kg/cm2 are quite common. The moisture content of the mash is reduced from 60-70 percent to about 50 percent. The pressing time can be as short as 15 minutes with the hydraulic press or as long as 4 days or more when stones are relied upon.
The cake obtained after pressing needs to be broken down into granules. This can be done manually or mechanically by passing it again in a grating machine. The powdery granules obtained can then be further processed into the desired products.
2.2.2 Special requirements
Cyanogenesis and safety issues
The single most important constraint in the expansion of cassava utilisation is its association with cyanide. It is essential that this association be well understood for the promotion of the crop.
Cyanogenesis, the ability of plants to produce, under some circumstances, the toxic hydrogen cyanide (HCN), exists in over 2000 plant species belonging to more than 100 families. In all species so far examined, HCN is never produced and stored at any stage of plant growth. The plants produce complex compounds, mainly glucosides, but in some case lipids, which may break down to produce HCN. Those compounds are therefore known as cyanogenic compounds. Plants also produce enzymes that break down the cyanogenic compounds but they are both always stored separately inside plant cells. It is only when the plant is damaged, and the structural integrity of the plant cells is destroyed that the enzyme acts on the cyanogenic compounds to produce cyanide.
Cassava produces two cyanogenic glucosides, linamarin and lotaustralin, in about 10 to 1 ratio. The amino acids valine and isoleucine are the precursors used in the synthesis of linamarin and lotaustralin respectively. The metabolic pathway for converting valine to linamarin has been elucidated by Koch et al. (1992).
In cassava plant cells, the cyanogenic glucosides are stored inside the vacuoles in the cytoplasm while the enzyme capable of degrading them is located in the cell wall outside the cytoplasm (Mpkong et al, 1991). Therefore, in intact cells the breakdown of cyanogenic glucosides would not occur. When cassava tissues are bruised and the cellular structures are disrupted, linamarin and lotaustralin come in contact with linamarase and are degraded.
The breakdown of linamarin leads to the formation of acetone cyanohydrin and glucose (Figure 3). At pH above 5, the acetone cyanohydrin will spontaneously break down into acetone and HCN. This breakdown may also be catalysed by the enzyme hydroxynitrile lyase (HNL) which is also present in cassava. Once HCN is produced, it will dissipate in the air (since its boiling temperature is 25.7oC). In damaged plant tissues, which includes processed roots and leaves, it is possible to find non-hydrolysed cyanogenic glucosides, cyanohydrins and traces of HCN. The term cyanogen refers to any of these three compounds.
Old analytical methods for quantifying cyanogens found in the literature often have the shortcoming that they do not achieve complete hydrolysis of cyanogenic glucosides, and therefore, under-estimate. A breakthrough was achieved with the method developed by Cooke (1978). In this method, all the cyanogens are extracted from the sample under conditions that stabilise them, and are quantitatively converted to cyanide ions that are specifically measured by a modified Epstein reaction. The entity measured in this fashion is termed "total cyanogen content". Modifications of the Cooke's method (O'Brien et al., 1990; Essers et al., 1993) have now made it possible to distinctively quantify hydrocyanic acid, cyanohydrins and cyanogenic glucosides.
Figure 3. Enzymatic hydrolysis of linamarin
The term "free cyanide" is used by some authors to refer to hydrocyanic acid and by others to the sum of hydrocyanic acid and cyanohydrins. Some authors use the term "bound cyanide" to refer to cyanogenic glucosides, while others may use it to refer to hydrocyanic acid bound to albumin and other blood proteins as part of in vivo cyanide detoxification processes. In the case of total cyanogen content defined above, what this value represent is the maximum amount of HCN that could be obtained from a sample. It therefore represents the CYANOGENIC POTENTIAL (sometimes abbreviated as CNP) of the sample and is usually expressed as mg HCN-equivalent per 100 g, or per kg of sample, taking care to specify whether the value is expressed on fresh or dry weight basis. Because of its simplicity, safety and low cost of reagents used, the method proposed by Essers et al. (1993) is recommended.
Cooke (1978) has studied the stability of the cyanohydrin. He found that at 30C and pH 6 it had a half-life of about 30 minutes, and that alkaline pH favoured its dissociation, while acid pH favoured its stability. This is important for the quantification of cyanogens and for the interpretation of cyanogen content of cassava reported in the literature.
Cyanogenic glucosides are not uniformly distributed in the various tissues of cassava plants. The largest concentration is usually found in the peel's cortex, and the lowest in the central pith; the leaves often contain the next highest concentration (De Bruijn, 1971). Younger tissues contain more total cyanide than older ones. In the root, the section closest to the stem (proximal) contains more total cyanide than the middle and distal sections; there is a shallow longitudinal gradient from the proximal to the distal end. From the peel side of the central pith to the centre of the root, the cyanogenic glucosides gradient is more pronounced; the concentration of cyanogenic glucosides is greatest in the outermost 2-3 mm layer and drops sharply towards the centre (Kojima et al., 1983).
Among 67 varieties analysed by de Bruijn (1971), the cyanogenic potential varied from 31 to 630 mg/kg in the root (fresh weight) and from 540 to 1450 mg/kg in the leaves (fresh weight). Similar ranges of cyanogenic potential were found in larger collections of varieties at the International Institute of Tropical Agriculture (IITA) in Nigeria (851 genotypes) and at the Centro Internacional de Agricultura Tropical (CIAT) in Colombia (560 genotypes) (Bokanga, 1994). No correlation was found between the total cyanogenic potentials of roots and leaves. Recent and old investigations have also confirmed this lack of correlation (Bokanga, 1994; Cooke et al., 1978a).
Cassava plants are arbitrarily classified into low- and high-cyanide varieties depending on the cyanogen content of their roots: low-cyanide varieties having roots with less than 100mg HCN-equivalent per kg (fresh weight), and the roots of high-cyanide varieties being above that figure (Hahn and Keyser, 1985). This is not unrelated to the toxicity classification proposed by Bolhuis (1954) in which cassava roots containing up to 50 mg HCN-equivalent per kg are considered innocuous, 50 to 100 mg HCN-equivalent per kg are considered moderately poisonous, and above 100 mg HCN-equivalent are considered dangerously poisonous. The scientific bases for these classifications have never been explained and required more investigation.
The organoleptic descriptors 'sweet' and 'bitter' are often used to characterise cassava varieties. Although earlier reports have associated bitter/sweet varieties with high/low levels of cyanogenic glucosides (Bolhuis, 1954), a cause-effect relationship has not been established (Coursey, 1973; Pereira et al., 1981). A bitter compound other than the cyanogenic glucosides has been isolated (King and Bradbury, 1996). Nevertheless, recent surveys in Africa have shown that farmers associate bitterness of cassava roots with toxicity (Chiwona-Karltun, in press).
Cassava consumption and health
Reported toxic effects of cassava are relatively rare in comparison with its wide use as a staple. A comprehensive review on this topic has been published (Bokanga et al., 1994). High and continuous consumption of cassava has been associated with various diseases and nutritional disorders: tropical ataxic neuropathy (Osuntokun, 1972), goitre and cretinism (Ermans et al., 1983), spastic paraparesis ( Mozambique Ministry of Health, 1984; Cliff et al., 1985) or konzo (Howlett et al., 1990). Contrary to the terminology used in earlier publications, there is no cyanide (HCN) of importance in cassava products. These contain variable amounts of cyanogenic glucosides and cyanohydrins. Upon consumption, cyanohydrins can readily decompose into cyanide, but cyanogenic glucosides are partly excreted unchanged in the urine. The cyanide produced is rapidly converted to thiocyanate by the enzyme rhodanese, which is widely distributed in the human body, with the highest concentration being in the liver and kidneys (Auriga and Koj, 1975). Thiocyanate has a known goitrogenic effect: it interferes with the ability of the body to use a limited supply of dietary iodine. However, a high thiocyanate load does not show a goitrogenic effect if the dietary iodine intake is adequate (Delange et al., 1994). Therefore, nutritionists should be aware of the potential goitrogenic effect of cassava in populations in tropical countries with marginal iodine supply and with cassava processing methods that are not efficient in reducing the cyanogen content of cassava food products.
There is increasing evidence to link prolonged consumption of insufficiently processed cassava with a newly described disease named konzo (Howlett, 1994; Tylleskar, 1994). Konzo is a paralytic disease (previously known as endemic spastic paraparesis) of abrupt onset appearing in very poor rural communities whose diets almost exclusively consist of bitter cassava roots. According to Tylleskar (1994), there are three prerequisites for the occurrence of konzo; a farming system dominated by bitter cassava, insufficient cassava processing that leaves high residual levels of cyanogens in cassava foods, and a protein deficient diet. Populations growing bitter cassava usually know how to process cassava into safe products (Dufour, 1994), and meeting one's protein requirements is a major priority in all communities. This explains why the occurrence of konzo is so rare and tends to be associated with agroecological disasters such as severe droughts (Howlett et al., 1990), with civil strife (Cliff, 1994) and with economic disturbances (Banea et al., 1992). It should also be emphasised that for millions of consumers, well-processed cassava is a staple food with no associated negative effects.
The first post-harvest task is transportation from the site of production and harvest to the site of processing and utilisation. Tshiunza et al. (1997) estimated that, on average, 70 person-days are needed to carry the harvest from one hectare of land (about 12 tons) over a distance of 1.5 km. The study which covered the six major African cassava producing countries (Cote d'Ivoire, D.R. Congo, Ghana, Nigeria and Tanzania) revealed that 85 percent of the farm output is carried directly to farmers' homes, 10 percent directly to market places and 5 percent to processing places. Transportation from field to home is by way of motor vehicle (15 percent), bicycles (9 percent), carts (6 percent), but mostly by head-load or back-load (70 percent). Women represent 81 percent of people involved in cassava transportation; they carry cassava to all destinations, while men's transportation is almost exclusively directed to the home (Nweke et al., in press). Harvesting and transportation are the most labour-intensive activities in cassava production; together they account for about 50 percent of labour needs for cassava production.
Reducing their moisture to a point where all physiological reactions and microbial growth are inhibited can tremendously increase the short shelf-life of cassava roots. In cassava, this point is at 14 percent moisture content, corresponding to a water activity of 0.70. The removal of moisture from cassava roots can be accomplished either by drying in the sun or in an oven. The most common method of drying cassava is sun-drying; moisture content is usually brought down to 8-12 percent. Cassava chips or granules from a grater are spread on a drying surface exposed to sunrays. The more chips on a drying surface, the slower the drying rate will be. Thin chips dry faster than thicker ones. It should be noted that the quality of the chips (e.g. starch content, white colour) is higher if the drying time is short. However, the cyanogenic potential of cassava decreases when the drying time is longer. Therefore, drying parameters that affect the drying rate, especially the loading rate (weight of drying material per unit area of drying surface), are important in determining the residual cyanogen content of the dried cassava.
2.8 Storage systems for cassava
The high perishability of cassava roots has prompted cassava consuming populations to develop storage schemes that alleviate the problem. There are reports that, 300 years ago, Amazonian Indians successfully stored fresh cassava roots by burying them in the soil, and that, in Mauritius 250 years ago, fresh cassava roots were stored in straw-lined trenches for periods of up to 12 months (Booth and Coursey, 1974). Inspired by these reports, researchers CIAT developed a clamp storage system similar in design to the European potato clamp (Cock, 1985; Richard and Coursey, 1981).
In this system, a conical pile of 300-500 kg of fresh cassava roots is seated on a circular bed of straw and covered with more straw. The whole unit is covered with soil to a thickness of 10 - 15 cm, the soil being dug from around the clamp so as to form a drainage ditch. With this storage system, acceptable levels of loss (0 - 20 percent) were achieved for periods of up to 2 months.
It was noticed during this storage time that bruised or otherwise injured roots tend to undergo a wound-healing response that prevent vascular discoloration or reversed it. This "curing" was correlated with a resistance to discoloration by application of exogenous scopoletin (Wheatley and Schwabe, 1985).
However, clamp storage performs less well during the hot season. The temperature inside the clamp easily reaches 40oC, and heavy losses result even after 1 month of storage (Booth and Coursey, 1974). It has been reported by Marriott et al. (1979) that pruning of cassava plants by removing the top of the plant and leaving a short (20 cm) leafless stem 2 to 3 weeks before harvest resulted in roots resistant to primary deterioration even if the roots are severely damaged. These authors have put forth that this resistance was suggestive of a control mechanism for vascular streaking dependent on a factor (or factors) produced in the leaves and translocated to the roots. In accordance with this hypothesis are the findings of Wheatley and Schwabe (1985) that pruning reduces scopoletin accumulation in the roots but not the response to exogenous scopoletin.
The clamp storage system is not compatible with transportation. To circumvent this, storage in boxes was designed (See Figure 4). Cassava roots are packed in boxes containing adsorbent material such as sawdust (Rickard and Coursey, 1981). The relative humidity inside the box is critical for a successful storage: too high, deterioration due to bacteria and fungi rapidly sets in; too low, vascular deterioration is not prevented.
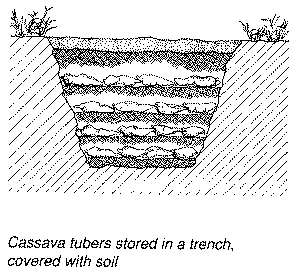
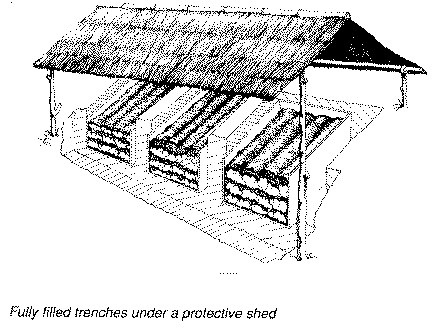
Figure 4. Storage systems for fresh cassava roots.
Packing cassava roots in polyethylene bags was tried and shown to preserve the roots for about 2 months (Cock, 1985). However, complete loss of the stored roots occurred as a result of microbial deterioration. Treating the roots with fungicides retarded the onset of spoilage (Rickard and Coursey, 1981).
The deterioration of cassava can be greatly reduced by cold storage. When kept below 4oC, cassava roots do not show internal discoloration. They still, however, remain susceptible to spoilage by fungi (Rickard and Coursey, 1981). The same authors report that cassava roots could be kept satisfactorily under deep-freeze conditions but that changes in texture occurred in stored samples. Deep freezing of cassava has received little attention from researchers, probably due to the rationale that high-cost storage methods were not suitable for a low-cost commodity such as cassava.
The storage of processed cassava products presents fewer problems than the storage of fresh roots, especially when these products have low moisture content. The major causes of losses are insect pests and fungi (Ingram and Humphries, 1972). A survey of cassava chips processing areas of Benin, Ghana and Nigeria has indicated that the most common fungi were Rhizopus sp. (47.5 percent of total samples) and Aspergillus sp. (29.6 percent) (IITA, 1996). Fungi proliferate when the moisture content of cassava chips exceeds 14 percent. A large majority of the samples in all three countries had moisture content below the critical moisture level.
The Environmentally Sound Cassava Plant Protection (ESCaPP) project of the IITA has determined that the main insect feeding on dry cassava chips in Benin Republic was Dinoderus sp. (Saizonou, 1996). Other insects of importance belong to the species Carpophilus sp., Araecerus fasciculatus and Rhizopertha dominica. Recently, the large grain borer, Prostephanus truncatus , a storage pest of maize, has been found infesting cassava chips in storage particularly during the rainy season. Infestation by all insects is heavier in the rainy season than in the dry season, is more prevalent in the humid zone than in the savannas, and is found more in large chips than in smaller ones (Dossou, 1996). Maximum infestation was found after 6 to 8 months in storage, at which time chips would fall into dust when squeezed.