TECHNICAL PAPER #25
UNDERSTANDING BATTERIES
By
Lee Merriman
Technical Reviewers
J.F. Douglas
James H. Hahn
Lester H. Smith, Jr.
Published By
VITA
1600 Wilson Boulevard, Suite 500
Arlington, Virginia 22209 USA
Tel: 703/276-1800 . Fax: 703/243-1865
Internet: pr-info@vita.org
Understanding Batteries
ISBN: 0-86619-225-5
[C]1985, Volunteers in Technical Assistance
PREFACE
This paper is one of a series published by Volunteers in
Technical
Assistance to provide an introduction to specific
state-of-the-art
technologies of interest to people in developing countries.
The papers are intended to be used as guidelines to help
people choose technologies that are suitable to their
situations.
They are not intended to provide construction or
implementation
details. People are
urged to contact VITA or a similar organization
for further information and technical assistance if they
find that a particular technology seems to meet their needs.
The papers in the series were written, reviewed, and
illustrated
almost entirely by VITA Volunteer technical experts on a
purely
voluntary basis.
Some 500 volunteers were involved in the production
of the first 100 titles issued, contributing approximately
5,000 hours of their time.
VITA staff included Maria Giannuzzi
as editor, Suzanne Brooks handling typesetting and layout,
and
Margaret Crouch as project manager.
The author of this paper, VITA Volunteer Horace McCracken,
is the
president of the McCracken Solar Company in Alturas,
California.
The co-author, VITA Volunteer Joel Gordes, is currently the
solar
design analyst for the State of Connecticut's Solar Mortgage
Subsidy Program. The
reviewers are also VITA volunteers.
Daniel
Dunham has done consulting in solar and alternative sources
of
energy for VITA and AID.
He has lived and worked in India, Pakistan,
and Morocco. Mr.
Dunham has also prepared a state-of-the-art
survey on solar stills for AID.
Jacques Le Normand is Assistant
Director at the Brace Research Institute, Quebec, Canada,
which does research in renewable energy.
He has supervised work
with solar collectors and has written several publiations on
solar and wind energy, and conservation.
Darrell G. Phippen is a
mechanical engineer and development specialist who works
with
Food for the Hungry in Scottsdale, Arizona.
VITA is a private, nonprofit organization that supports
people
working on technical problems in developing countries.
VITA offers
information and assistance aimed at helping individuals and
groups to select and implement technologies appropriate to
their
situations. VITA
maintains an international Inquiry Service, a
specialized documentation center, and a computerized roster
of
volunteer technical consultants; manages long-term field
projects;
and publishes a variety of technical manuals and papers.
UNDERSTANDING BATTERIES
By VITA Volunteer Lee Merriman
I. INTRODUCTION
Batteries have been in use for many years, but today there
is a
greater demand for battery power than ever before.
This renewed
interest has been brought about not only by new developments
but
also by the diversity of uses for batteries in civilian,
industrial,
and military applications.
This paper provides a basic understanding of batteries and
traces
their development from the early 1800s to the present
day. Research
and development continues in an effort to solve the inherent
weakness of batteries, namely, how to pack more energy
into a smaller package.
An electric cell or battery is a device that transforms the
chemical energy contained within its active materials
directly
into electrical energy by means of an electrochemical
reaction.
This type of reaction involves the transfer of electrons
from one
material to another through a conducting solution.
Historically,
batteries played an important role in the early days of
electrical
development both in the United States and in Europe.
In 1800 an Italian scientist named Volta discovered that by
immersing two dissimilar conductors in a chemical solution
an
electromotive force (EMF) or voltage was established between
the
two conductors.
Figure 1 illustrates a simple Voltaic cell.
ub1x1.gif (393x393)

The solid conductors of the cell are called electrodes and
the
conducting liquid the electrolyte.
A cell consists of two electrodes
and an electrolyte.
A battery consists of one or more
cells. The voltage
of the cell depends upon the material of the
electrodes and the electrolyte.
The electric current output and
the power of the cell are dependent upon the plate
dimensions and
the weight of the electrode material.
There are two general types of batteries in use today: the
primary
type or "dry cell" and the secondary storage
battery. A
primary battery produces a current by discharge action when
one
of the electrodes of the cell is decomposed during use.
This type
of cell cannot be restored to use again by recharging and
the
entire cell must be discarded when it is no longer
active. Secondary
cells, on the other hand, are chemically reversible and can
be charged and discharged over many cycles of operation
before
being replaced.
In the simple voltage cell shown in Figure 2, when two
dissimilar
ub2x3.gif (486x486)

metals, zinc and copper, are suspended in an electrolyte of
dilute sulfuric acid, a potential of approxiamtely 1.10 volts
will exist between the electrodes.
The zinc electrode will be
negative and the copper electrode will be positive.
When the
switch in the external load circuit is closed, a current
will
flow through the load (energy-absorbing device) and battery
in
accordance to Ohm's Law.(*) As the load current continues to
flow,
hydrogen as bubbles will appear and cover the copper plate,
and
the zinc plate will gradually dissolve.
The main disadvantage
with this cell is that the gas bubbles increase the internal
resistance of the cell, causing current output to decrease.
----------------------
(*) The direct current flowing in an electrical circuit is
directly
proportional to the voltage applied to the circuit.
The constant
of proportionality R, called the electrical resistance, is
given
by the equation V = RI, in which "V" is the
applied voltage and
"I" is the current.
II. TECHNOLOGY VARIATIONS
PRIMARY BATTERIES
Several different types of primary-type wet cells were
developed
and used in the United States.
Most notable among these were the
gravity cell, the caustic-copper oxide cell, the
air-depolarized
cell, and the Lelanche cell.
Each cell had its own operating
characteristics, and current capacities ranged from less
than one
ampere (amp) for the Lelanche cell to several hundred
amperes for
the caustic-copper oxide cell.
The British Post Office developed
a wet cell known as the Daniel's cell, which offered several
outstanding operating features.
There were two main difficulties with the primary-type cell
construction, deterioration by local action and cell
polarization.
Local action is an internal chemical action inherent to
batteries; the life of the cell is gradually diminished even
though no load is connected to its terminals.
Local action is
defined as the discharge of active material of either plate
due
to some impurity in the electrolyte or plate material.
This
action causes the formation of short-circuited cells, which
cause
the metal to deteriorate.
Cell polarization is caused by hydrogen bubbles being
deposited
on the cathode when current flows through the cell.
This lowers
the terminal voltage and increases the internal resistance
of the
battery. Various
methods for neutralizing this polarizing effect
were used, either by chemical or mechanical construction,
which
led to the development of the air-depolarized cell.
In the air-depolarized cell, the electrode was made of a
highly
absorbent form of carbon and was suspended above the
electrolyte
level. Since the
carbon electrode was not immersed in the electrolyte
solution, polarization of the cell was prevented.
In
operation, oxygen surrounding the porous surface of the
carbon
electrode combines with the hydrogen evolved at the surface
of
the carbon electrode and electrolyte.
Good ventilation was required
to maintain a satisfactory air supply for operation.
The
Edison carbon cell and the Carbonaire battery were
representative
of the air-depolarized type.
Wet primary-type cells have largely
been replaced by the secondary-type storage battery.
The modern day "dry cell," which was developed by
Georges
Lelanche in 1868, is a modification of the old Lelanche wet
cell.
The difference is that only sufficient water is added to the
electrolyte to moisten an absorbent lining.
The modern dry cell
is the most widely used of all primary batteries today
chiefly
because of their low cost, reliable performance, and
widespread
availability. Dry
cell batteries are made in ratings of 1.5, 3,
6, 7.5, 9, 22.5, 45, 67, and 90 volts.
The most common type of construction for a dry cell is shown
in
Figure 3.
ub3x4.gif (486x486)
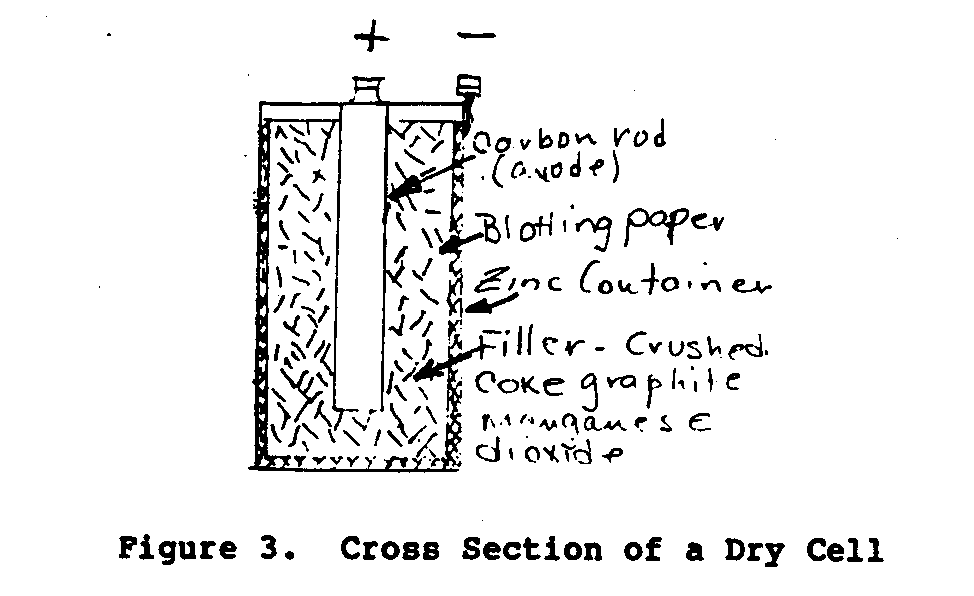
The cell in Figure 3 uses a carbon rod for the anode or
positive
terminal and an outside zinc container (case) for the
negative
terminal. The zinc
case has an inner lining of absorbent paper
material which is saturated with the electrolyte.
The space
between the electrodes is filled with a mixture of crushed
coke,
manganese dioxide, and graphite.
Manganese is added as a depolarizer.
The electrolyte is salammonic and zinc chloride.
The
top of the case is sealed with a sealing compound and the
zinc
container is enclosed in a paper container.
The voltage of a new
dry cell is 1.4 to 1.6 volts.
Dry cell batteries fall into three general classes: (1)
flashlight
batteries usually 1-1/4 inch in diameter and 2-1/2 inches
high with a current capacity of about 3 amp-hours; (2) large
size
cells, more commonly referred to as the Number 6 dry cell,
approximately
2-1/2 inches in diameter and 6 inches high with a
current rating of about 30 amp-hours; and (3) the "heavy
duty"
and high voltage types, which might be one cell or a
combination
of cells, used in industrial service with current capacities
of
50 amp-hours or greater.
The ampere-hour capacity is the rate of
discharge a battery can maintain for a given period of time,
usually eight hours.
For example, a 30 amp-hour rated battery
normally could supply about 3-1/2 amps for eight hours.
As ordinarily
used, however, dry cells provide less than their rating.
The shelf life is limited by local action and for that reason
some manufacturers stamp a service date on the outer
covering of
each cell. Local
action causes eventual deterioration of the
battery, and after about one or two years storage, the
battery
becomes useless.
Since the zinc electrode forms part of the outer
wall, its gradual destruction weakens the cell structure,
and as
the developed hydrogen gas builds up internal pressure, it
can
rupture and spill its corrosive contents.
For this reason, equipment
should never be stored with dry cells over long periods of
time. Dry cells
require no maintenance and when they no longer
operate are discarded and replaced.
A more recent type of dry cell developed is the Ruben or
Mercury
cell (Figure 4).
This cell was developed during World War II by
ub4x6.gif (600x600)

Ruben Laboratories and P.R. Mallory Company for operating
small
electronic equipment requiring high current power.
This cell is
made in two forms: the "roll anode" and the
"button type." The
anode is amalgamated zinc and the cathode is a mercuric
oxide
depolarized material mixed with graphite.
The electrolyte is a
solution of potassium hydroxide (KOH) containing potassium
zincate.
These cells are far superior to the Lelanche dry cell owing
to their compact size, flat voltage characteristic, and very
long
shelf life. The
no-load voltage of these cells is 1.34 volts.
Several advanced developments have been made in small
batteries,
both primary and secondary-type cells, which include the
magnesium, alkaline, silver-zinc, and lithium.
Table 1 lists the
ubxtab1.gif (600x600)

characteristics and applications of these cells.
SECONDARY STORAGE BATTERIES
Since 1965, there has been renewed interest in using storage
batteries in power systems.
This is because modern power consumption
involves very uneven load demands and increasing peak load
demands. When a
system must deliver more power (increase in load
demand), the supplier can meet the demand by either
switching an
additional generator onto the system or switching a charged
battery bank onto the line.
The latter requires a much smaller
investment.
The revival of batteries as power system units primarily has
begun with small independent systems such as wind- or
water-driven
generators. In such
systems, storage batteries perform
two important functions.
First, during periods of low load demand,
the system battery can store much of the generated energy,
which would otherwise be lost to the system.
Second, energy
stored during the off-peak period is available during times
of
peak load demand.
The importance of the latter can be illustrated
with the following quantitative example: Suppose the
capacity of the battery has a discharge power rate equal to
half
of the generator power capacity ([P.sub.B] = 0.5
[P.sub.G]). This means that
under normal conditions, during periods of high load demand,
the
generator-battery combination can for several hours serve a
load
of up to 1.5 times what the generator alone could serve.
Another reason for the increased interest in secondary
storage
batteries is the need for backup power for some of the newer
technology. For
example, most modern computers involve some form
of "volatile" storage of information, that is, the
information is
lost if power is removed.
To guard against this possibility, many
computer systems use "uninterruptible" power
systems, based on
storage batteries, to supply electrical current to the
computer
equipment when commercial power is lost.
The storage battery, constructed with secondary wet cells,
is
similar in action to a primary cell, except the chemical
actions
involved are practically completely reversible.
Once the cell is
discharged, current from an external source, passed through
the
cell in the opposite direction, will substantially restore
the
battery to its original charged condition.
There are three types of storage batteries currently
available:
(1) the lead-acid type; (2) the nickel-iron or alkaline
battery
(Edison cell); and (3) the nickel-cadmium or alkali-type
(Nicad).
Lead-Acid Batteries
The lead-acid battery is the most widely used type of
battery
today because of its low cost, reliability, good performance
characteristics, and wide application.
This battery is manufactured
in many sizes and capacities ranging from 1 amp-hour up to
several thousand amp-hours rating.(*)
The storage cell uses reactive sponge lead for the negative
electrode (Pb), lead dioxide for the positive electrode (Pb0
),
and dilute sulfuric acid for the electrolyte.
The electrode
materials have little structural strength and must be
supported
on plates or grids.
The grid of the battery plate has two functions:
first, it supports the active plate material; and second,
it serves as a conductor to connect the plate terminal to
all
parts of the active material.
Lead storage battery plates are divided into two types, the
Plante (formed) and the Faure (pasted), as shown in Figure
5. In
ub5x9.gif (600x600)

the Plante-type of construction the active material is
electrically
formed of pure lead by an electrochemical process from the
metallic lead of the supporting grid.
In the Faure-type the
active material is applied to the supporting grid in the
form of
a paste follwed by a setting, drying, and forming operation.
Figure 5 shows the Plante (A) and Faure (B) lead cell
plates. The
cell assemblies are soldered together to form positive and
negative
groups which are interleaved together to make up the
complete
battery cell.
Separators are placed between the electrodes,
and the complete element is placed in a container and
sealed. The
use of large plates with close spacing limits the internal
resistance
of the battery to a low level.
Figure 6 shows a cutaway
ub6x9.gif (600x600)

view of the lead storage cell.
During discharge the battery material of both plates is
converted
into lead sulfate.
The amount of lead sulfate formed onthe plates
and the amount of acid lost from the electrolyte are in
exact
proportion to the rate of discharge.
The reverse action takes
place when the cell is charged.
Cell chemical reactions are
represented by the following equation; however, this is a
simplified form as the actual action is much more complicated.
-----------------------
(*) Battery ampere-hour rating is normally based upon an
8-hour discharge
rate.
At the positive plate:
Pb[O.sub.2] +
HS[O.sub.4][sup.-] + [3H.sup.+] + [2e.sup.-](*) -----> PB[SO.sub.4] +
2[H.sub.2]O
At the negative plate:
Pb + HS[O.sub.4][sup.-] -----> Pb[SO.sub.4] + [H.sup.+] +
[2e.sup.-]
The combined cell reaction for both discharge and charge is
expressed
by the following equation:
discharge
------------->
Pb[O.sub.2] + Pb + 2[H.sub.2] S[O.sub.4] <======
2Pb[SO.sub.4] + 2[H.sub.2]O + electrical energy
sulfuric
plate
plate acid
plates
<-------------
charge
On discharge the acid separates from the electrolyte and
forms a
chemical combination with the plates, changing it to lead
sulfate.
As discharge continues, additional acid is drawn from the
electrolyte until current will cease to flow.
The water, formed
by the loss of acid to the plates, lowers the remaining
specific
gravity(**) of the electrolyte.
In common practice, discharge is
always stopped before the plates have entirely sulfated,
because
once entirely sulfated, battery condition cannot be
converted
back to active material on charge.
On charge the reverse action
takes place: the acid in the sulfated plates is driven back
into
the electrolyte, and the S[O.sub.4] combines with hydrogen
in the water
to form additional sulfuric acid ([H.sub.2][SO.sub.4]).
Electrolyte for lead-acid cells is dilute sulfuric
acid. For a
fully charged battery the specific gravity varies from 1.200
to
1.30 and when discharged 1.150 (pure water measures
1.00). The
specific gravity is measured by a syringe-type hydrometer as
shown in Figure 7, and values are temperature corrected.
ub7x11.gif (600x600)

------------------------
(*) The symbol e- stands for electrons.
(**) Specific gravity is defined as the ratio of weight of a
given
volume of a substance to an equal volume of pure water.
The voltage of a lead cell is approximately 2.10 volts at no
load
but is higher when being charged.
Normal voltage on charge is
2.15 volts and as the cell approaches full charge this value
rapidly increases to between 2.5 and 2.6 volts.
This later interval
of charge is known as the "gassing period."
Gassing of the
electrolyte at any time during charging should be avoided as
the
charge rate is too high.
As a cell reaches its final fully
charged condition, a high current is not advisable as this
excess
current decomposes the water in the electrolyte, which is
driven
off in the form of gas.
The lead-acid battery has several disadvantages:
(1) cells are
temperature sensitive and lose power in cold temperatures;
(2)
cell plates tend to buckle and distort on sustained, high
current
service, and (3) special care must be observed when a
battery is
not used for long periods, otherwise the cells will sulfate.
Nickel-Iron Batteries
The nickel-iron or alkaline battery was developed to
overcome the
inherent disadvantages of the lead-plate cell.
It is a radical
departure from it in both construction and operation.
In the
United States this battery is known as the "Edison
cell," named
after its inventor Thomas A. Edison.
Figure 8 shows the construction
ub8x13.gif (600x600)

of a typical cell. The
positive plate consists of steel
tubes containing nickel hydrate and nickel added in
alternate
layers. The negative
plate is formed of flat steel boxes or
pockets which are perforated and packed with iron oxide
granules.
Sheet-steel grids support these tubes and pockets, which are
bolted together to form positive and negative cell
groups. Cell
terminals and the steel container are nickel plated.
All separators
and insulating parts are made of rubber.
The cell uses an
electrolyte of 21 percent solution of caustic potash
containing a
small amount of lithium hydrate.
The chemistry of this cell is quite complicated, and the
chemical
reaction occurring inside the cell is entirely different
from
that of the lead cell.
The electrolyte acts merely as a conducting
medium and does not enter into combination with any of the
active plate material during operation.
Its specific gravity
remains practically constant over the complete cycle of
charge
and discharge.
Condition of battery charge or discharge is determined
by a voltmeter reading and not by the specific gravity of
the electrolyte. The
alkaline battery cell reaction is:
discharge
------------------>
[Fe.sub.2] + 2NiOOH + KOH + 2[H.sub.2]O ------->
[Fe.sub.2][(OH).sub.2] + 2Ni[(OH).sub.2] + KOH + electrical
<------
<-----------------
energy
charge
The voltage of each cell is approximately 1.50 volts on open
circuit, but is higher on charge and lower under load
conditions.
These batteries are given an ampere-hour capacity rating
based
upon their rate of discharge up to the final voltage of 1.00
per
cell. Some current
ratings are based upon a 5-1/2-hour continuous
discharge rate, while others are based upon a 3-1/2-hour
rate.
Unlike the lead-cell battery, there is no minimum voltage
below
which this type of cell cannot be discharged.
In fact, this cell
can be discharged to zero volts, short-circuited at its
terminals,
and left in this condition for an indefinite period.
This
is the method by which an alkaline battery is put into
storage.
Also, this cell can be accidentally overcharged, charged in
the
wrong direction, and momentarily short-circuited without
harm.
Alkaline batteries are not injured by freezing and an
electrolyte
with a specific gravity of 1.200 at 15.5[degrees]C
(60[degrees]F) freezes solid
at -66[degrees]C (-87[degrees]F).
The electrolyte of this cell gradually deteriorates
during use and must eventually be changed.
The main advantages of the nickel-iron cell are: (1) it is
extremely light and strong owing to its steel construction;
(2)
it offers an indefinitely long life; and (3) it overcomes
the
cell sulfating problem of the lead-acid battery.
The chief
disadvantage is its high first cost and high internal
resistance.
Nickel-Cadmium Batteries
Nickel-cadmium or Nicad batteries, a relatively new addition
to
storage cells, were developed in Europe.
These batteries consist
of interleaved assemblies of positive and negative plates
mounted
in a sealed steel container.
The positive active material, nickel
hydroxide, and the negative active material, cadmium oxide,
are
encased in identical, finely perforated steel pockets.
The plates
are made up of rows of these pockets, which are crimped and
formed into steel frames.
Positive and negative plate assemblies
are bolted together to heavy steel bus bars.
Plate groups are
interleaved and separated by thin plastic rods.
The cell electrical
terminals and case are nickel plated.
The electrolyte is a
solution of specially purified caustic potash (potassium
hydroxide)
dissolved in distilled water.
Figure 9 shows a cutaway view
ub9x15.gif (600x600)

of the Nicad battery.
The simplified cell reaction is:
charge
<-------------------
Cd + 2NIOOH + KOH + 2[H.sub.2]O ------> Cd[(OH).sub.2] +
2Ni[(OH).sub.2] + KOH + electrical
<------
energy
-------------------->
discharge
During charge or discharge of the cell, there is practically
no
change in the specific gravity of the electrolyte.
Like the
Edison cell, the sole function of the electrolyte is to act
as a
conductor for the transfer of hydrogen ions from one
electrode to
the other. The
voltage rating of each cell is 1.20 volts on open
circuit; when connected to an external load, this voltage
remains
fairly constant up to approximately 90 percent of its rated
capacity. The
amp-hour rating of the Nicad cells is based upon a
final discharge voltage of 1.10 volts per cell.
Unlike Edison
cells, Nicad batteries will be damaged by repeated
over-discharging
below their minimum cell rating of 1.10 volts.
Nicad batteries
have a temperature operating range from -51[degrees]C
(-60[degrees]F) to 93[degrees]C
(200[degrees]F).
Nicad batteries are vibration and shock resistant due to
their
steel construction; hold their charge well during long idle
periods; maintain a constant voltage source during
discharge; and
are not damaged by overcharge.
These batteries can be mounted in
any position on discharge.
Like the Edison cell, the Nicad battery
has a high first cost as compared with the lead-acid
battery;
however, this high cost is offset by their longer life
span. A comparison
of lead-acid, alkaline, and Nicad batteries is
presented in Table 2.
Table
2. Comparison of Lead-Acid, Nickel-Iron,
and Nickel-Cadmium Batteries
Operating Cell
Life
Typical Temperature
Energy
Charge/
Cell Range
Density
Discharge Cost
Type
Voltage ([degrees]C)
(Wh(*)/kg)
(Cycles) ($/Wh(*))
Lead-Acid
2.0 20 to 30
37
1200-1500 .08
Nickel-Iron 1.2
2.2 to 46
29
Nickel-Cadmium
1.25 (-51) to 93
33
(*)Watt-hours
General Maintenance Procedures for Storage Batteries
Proper maintenance is essential for continued trouble-free
service
of storage batteries.
While the cell construction is different
for the several types, maintenance is similar for all types
and consists of the following general procedures:
1.
Keep cells clean and dry;
2.
Check electrolyte level regularly;
3.
Keep batteries charged at all times; and
4.
Keep impurities of all kinds out of cells as
they will
have a
harmful effect and eventually ruin them.
Never
use any
tools or utensils (hydrometers, funnels, etc.)
that have
been used to service other electrolytes different
from that
required for that specific battery,
especially
tools used for lead-acid batteries.
5.
Refer to manufacturers' recommendations and
keep a
written
maintenance record.
The electrolyte of the lead-acid cell never requires
replacement
except for loss due to accidental spills.
However, in the Edison
and Nicad cells there is a gradual deterioration of their
electrolyte,
which must eventually be replaced over the life of the
battery.
BIBLIOGRAPHY/SUGGESTED READING LIST
Baumeister, T., ed. Mark's Standard Handbook for Mechanical
Engineers.
7th Edition. New
York, New York: McGraw-Hill Book
Company, 1967.
Carr, C.C. Craft's American Electrician's Handbook. 8th Edition.
New York, New
York: McGraw-Hill Book Company, 1961.
Fink and Batey.
Standard Handbook for Electrical Engineers. 11th
Edition. New
York, New York: McGraw-Hill Book Company, 1978.
Hubert, Charles I.
Preventative Maintenance of Electrical Equipment.
New York, New
York: McGraw-Hill Book Company, 1969.
Knowlton, A.E., Standard Handbook for Electrical Engineers.
8th
Edition. New
York, New York: McGraw-Hill Book Company, 1949.
McGraw-Hill Encyclopedia of Science and Technology. 5th
Edition.
New York, New
York: McGraw-Hill Book Company, 1982.
Timbre and Bush.
Principles of Electrical Engineering. 3rd Edition.
New York, New
York: Wiley and Sons, Inc., 1946.
Wolf, Stanley. Guide
to Electronic Measurement and Laboratory
Practices.
Englewood Cliffs, New Jersey: Prentice Hall,
Inc., 1977.
========================================
========================================

